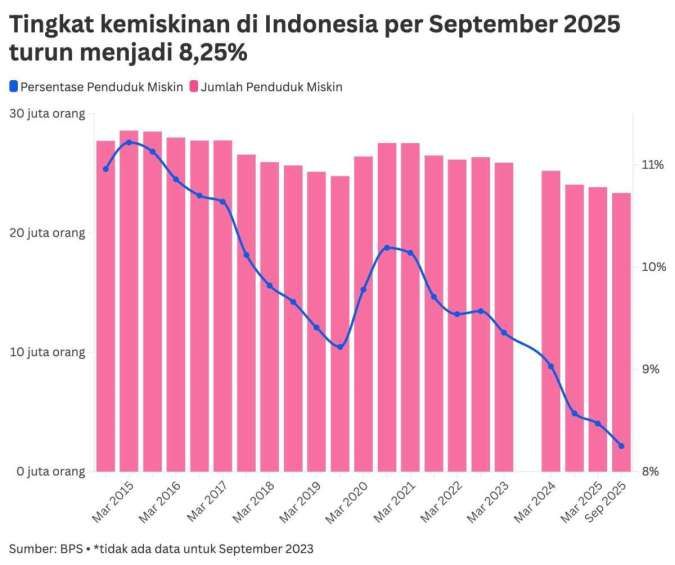
FDA to Discuss Pfizer and Moderna Requests for Covid Vaccines in Young Kids in June
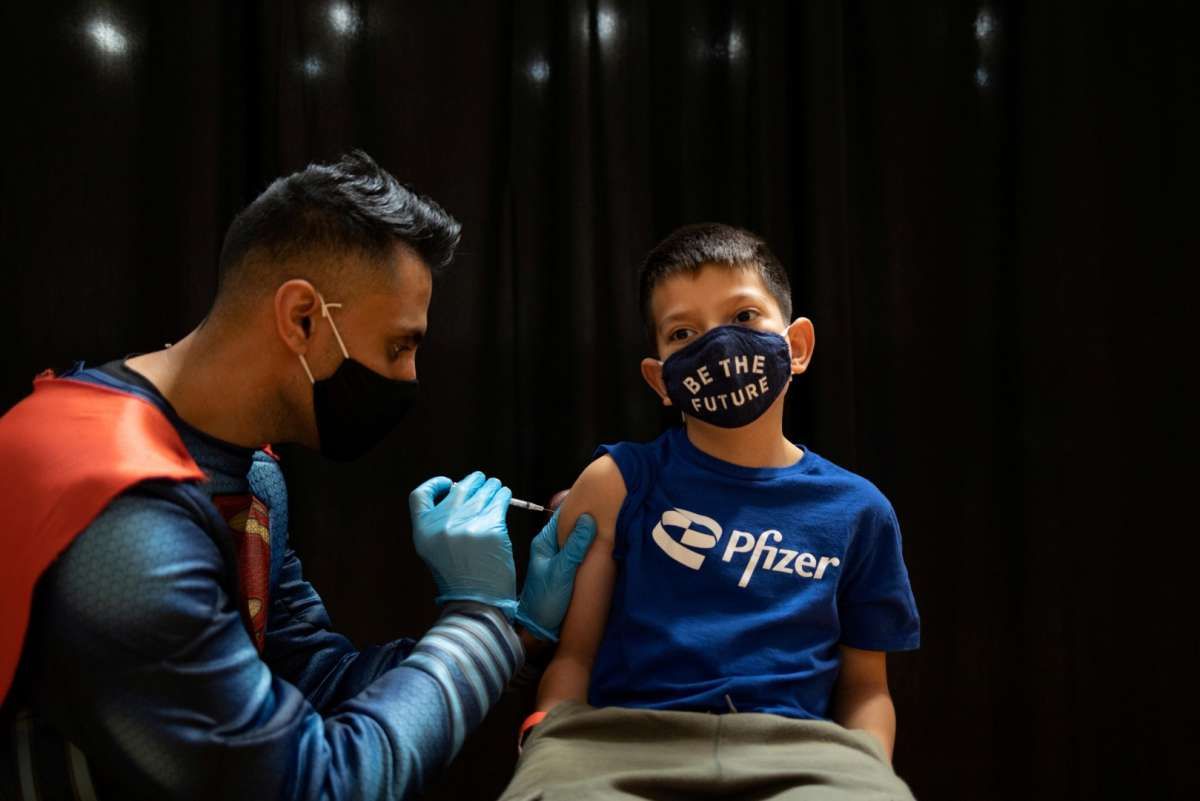
KONTAN.CO.ID - An advisory panel of experts to the US drug regulator will meet in June to review requests from Pfizer Inc and Moderna Inc for use of their Covid-19 vaccines in young children.The US Food and Drug Administration said on Friday.
The FDA said it would also convene a meeting to review Novavax's request for emergency use authorization of its vaccine in individuals aged 18 and older.